“By inhibiting thioredoxin reductase activity and increasing intracellular reactive oxygen species levels, auranofin induced a lethal endoplasmic reticulum stress response in cultured and primary CLL cells. In addition, auranofin displayed synergistic lethality with heme oxygenase-1 and glutamate-cysteine ligase inhibitors against CLL cells. Auranofin overcame apoptosis resistance mediated by protective stromal cells, and it also killed primary CLL cells with deletion of chromosome 11q or 17p. In TCL-1 transgenic mice, an in vivo model of CLL, auranofin treatment markedly reduced tumor cell burden and improved mouse survival. Our results provide a rationale to reposition the approved drug auranofin for clinical evaluation in the therapy of CLL”.
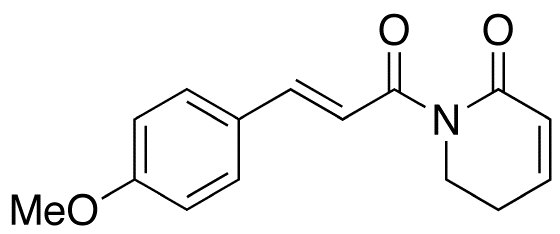
Auranofin Induces Lethal Oxidative and Endoplasmic Reticulum Stress and Exerts Potent Preclinical Activity against Chronic Lymphocytic Leukemia
Auranofin Induces a Reversible In-Vivo Stress Response That Correlates With a Transient Clinical Effect In Patients With Chronic Lymphocytic Leukemia
Effect of auranofin on oxidative and endoplasmic reticulum stress as well as anti-CLL activity with proteasome inhibitor
Targeting the Redox System to Overcome Mechanisms of Drug Resistance in Chronic Lymphocytic Leukemia
Phase I and II Study of Auranofin in Chronic Lymphocytic Leukemia (CLL)
New NIH Center Broadens Scope of Translational Research
Nearly 30 years after auranofin gained approval from the U.S. Food and Drug Administration to treat rheumatoid arthritis, researchers are repurposing the drug for a possible new use: chronic lymphocytic leukemia (CLL). Moreover, the arthritis drug could emerge as a model for accelerating patients’ access to other repurposed drugs or for rescuing drugs that the pharmaceutical industry has abandoned and now are languishing on companies’ shelves, researchers say.
“What we did was go from in vitro experiments directly into patients,” said Scott Weir, Pharm.D., Ph.D., director of the Institute for Advancing Medical Innovation at the University of Kansas Medical Center, one of several test sites nationwide, which soon will include the National Heart, Lung, and Blood Institute.
“We didn’t feel we needed to go through the traditional drug paradigm,” he said, given auranofin’s earlier testing for safety and efficacy.
As a result, less than 2 years after scientists discovered that auranofin could kill CLL cells in the lab, researchers began dosing the first relapsed CLL patient in a clinical trial. That compares with, on average, 8–10 years to reach a similar stage in drug development for a new drug, according to Weir, one of several authors of a recent commentary in Cancer Research about the pilot project.
The repurposing of older drugs such as auranofin, as well as second looks at unapproved agents stuck in the regulatory pipeline, is part of an intense systematic approach to translational research embodied in the first new center at the National Institutes of Health in more than a decade. The National Center for Advancing Translational Sciences (NCATS), which replaced the National Center for Research Resources earlier this year, incorporates many of the former center’s programs.